KUALA LUMPUR, July 18 — The Health Ministry (MOH) through the Medical Devices Authority (MDA) has released a list of two COVID-19 self-test kits that have received conditional approval for import and distribution in the country.
Through an infographic shared on MOH’s Facebook account, the test kits can be sold at registered community pharmacies or health facilities.
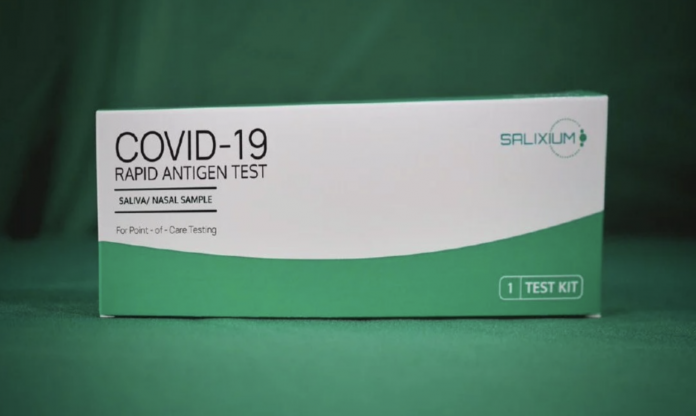
The two kits are Salixium COVID-19 Rapid Antigen Rapid Test (Saliva/Nasal Swab Samples) made by Reszon Diagnostic International Sdn Bhd Malaysia and Gmate COVID-19 Ag Saliva For Home Use by Philosys Co Ltd, South Korea.
MOH said the list will be updated from time to time, and it can also be checked on the MDA portal.