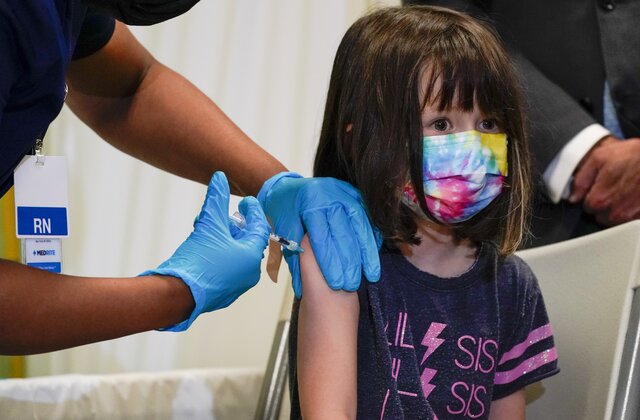
CANBERRA, Dec 5 — Australia’s medical regulator, the Therapeutic Goods Administration (TGA), on Sunday provisionally approved Pfizer’s coronavirus vaccine for children aged between 5 and 11.
Health Minister Greg Hunt said the federal government now expects the vaccine rollout for that cohort to start on Jan 10 pending approval from the Australian Technical Advisory Group on Immunisation (ATAGI).
“The vaccine dose approved by the TGA for children aged five to 11 is the same safe and effective vaccine used for other age cohorts, however is one-third the dose approved for those aged 12 and over,” Xinhua news agency quoted a media release from Hunt said.
“As with other age groups, the use of this vaccine in children aged 5-11 years should be given in two doses at least 3 weeks apart.”
There are approximately 2.3 million Australians in the 5-11 age bracket.
It added that as of Saturday, about 88 per cent of Australians aged 16 and over and 67.5 per cent of 12 to 15 year-olds were fully vaccinated against COVID-19.
On Sunday, Australia reported more than 1,200 new locally-acquired coronavirus cases and eight deaths as the country continues to battle the third wave of infections.
The majority of them — 980 cases and seven deaths were in Victoria, the country’s second-most populous state with Melbourne as the capital city.
The number of cases of the Omicron variant in Australia has increased to 18 after more cases were reported in the Australian Capital Territory (ACT) and New South Wales (NSW).
Parliament House in Canberra was closed on Sunday after a staffer to Greens leader Adam Bandt tested positive for COVID-19, it added.